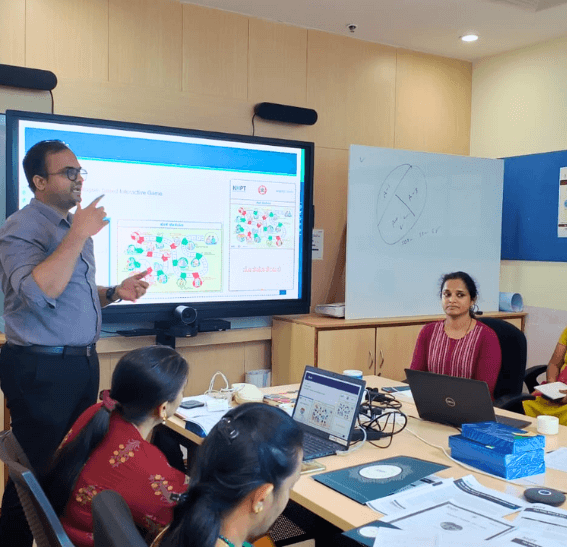
KHPT has a dedicated team pursuing impactful public health research focused on vulnerable communities including persons with TB , persons living with Non-Communicable Diseases, Persons living with HIV (PLHIV), adolescents, pregnant women and newborns, children, the elderly, and migrants.
We do not conduct any biomedical research or clinical research involving the administration of drugs or any such methods. The majority of our research proposals involve socio-behavioural studies and focus on behaviour change and health systems strengthening through mixed methods research and participatory research involving diverse stakeholders. Evidence generation and learning through robust research have informed programme design, implementation and evaluation.
KHPT’s Institutional Ethics Committee
The scope of KHPT Institutional Ethics Committee (KHPT IEC), or Ethics Committee (EC), as it is commonly called, will involve reviewing socio-behavioural research studies, research studies involving minimal risks and less than minimal risks, and research studies involving non-invasive or minimally invasive sampling collection procedures. KHPT EC will review both internal and external research studies. KHPT EC’s registration with the Department of Health Research, Ministry of Health & Family Welfare (Government of India) has been approved (EC/NEW/INST/2023/3255)